Tracks & Sessions
Track 1: Clinical research and clinical trials: an academic perspective
Clinical research is a branch of medical science that investigates the efficacy and accuracy of drugs, medical technologies, and treatment. This comprises systemic, observational, and experimental biomedical studies. Clinical research is important in disease prevention, treatment, diagnosis, and cure. This includes a series of events ranging from pre-clinical animal testing to various phases of drug development. Pre-clinical reviews and non-clinical investigations are a stage of pre-clinical research that occurs before to clinical trials. Treatment research comprises an intervention such as medicine, counselling, or new surgical methods. Prevention research seeks more effective strategies to avoid diseases and problems. Diagnostic research is the practise of looking for more effective approaches to identify disorder conditions. The goal of screening research is to discover better approaches to detect various diseases and health conditions. Quality of life trials look for ways to improve the comfort and quality of life of people who have a chronic illness. Genetic research aims to improve disorder prediction by determining and comprehending how genes and sickness may be related.
Track 2: Clinical trials conduct
Clinical trials are clinical research observations. Prospective behavioural study on humans to address specific questions about biomedical or behavioural therapies. This generates data on the safety and efficacy of the product. Multiple experimental treatments are tried in only one trial in the master protocol. The clinical trial protocol is used to describe and monitor the trial. This procedure includes a detailed research plan to ensure trail safety and health and to provide inspectors with a proper template for trail behaviour. A clinical trial involving novel medications is divided into five stages. Each stage of drug compliance is regarded as its own clinical study. The drug development process will progress through all four stages. Phase 0 - human pharmacodynamics and pharmacokinetics (testing on 10 to 15 participants). Phase 1 - safety assessment (testing in a small group of 20 to 80 participants). Phase 2 - determining the drug's efficacy (testing with a wider group of 100 to 300 people). Phase 3 consists of final validation of safety and efficacy (testing with a large group of participants ranging from 1000 to 3000). Phase 4 -safety studies during sales (post-marketing studies describe new information such as the treatment's risks, benefits, and recommended use).
Track 3: Clinical data management
Clinical data management ensures that data is collected, integrated, and available at an appropriate quality and cost. It leads to the generation of high-quality, dependable, and statistically sound clinical trial data. The data management plan describes the tasks to be performed in the data processing process and is used to describe data sources, data handling processes, and data control procedures. Clinical trials employing both an electronic CRF database design and a paper CRF. Validation rules are computerised analytic procedures that ensure the integrity and consistency of clinical trial data. Data entry is moving out towards the inspection site where the clinical trials are done using an electronic CRF. Samples obtained during clinical trials are sent to a single central laboratory for analysis. The CRF gathers adverse events reported during the course of the clinical trial; there is a distinct protocol in place to ensure that major adverse events are reported as soon as possible. The clinical data manager has responsibility for ensuring that data is reconciled between different processes.
Track 4: Clinical studies on various disorders
Clinical trials for distinct drugs and problems are intended to evaluate at least one medication for treating an infection, disorder, and moreover uncovering appealing to maintain the improvement or repeat of a disease or condition. This involves, among other things, remedies, antibodies, and lifestyle adjustments. Clinical trials for behavioural, mental, eating, and sleeping issues. Diabetes and cardiovascular disease clinical trials, Alzheimer's disease clinical trials, Trials on pulmonary/respiratory illnesses, Wounds and traumas, acquired immune deficiency syndrome, gynaecological infections, infectious colitis, and HIV infections are all being researched and studied.
Track 5: Ethics in clinical research
The essential ethical principles of various ailments related to research and scientific study are addressed in the field of research ethics. Medical ethics is a set of moral concepts that apply values to clinical treatment and scientific study. Medical ethics is built on a set of ideals that practitioners can refer to in the event of confusion or conflict. Privacy for research participants is a concept in research ethics that asserts that a person in human subject research has a right to privacy when participating in research. Shared decision-making in medicine (SDM) is a method in which both the patient and the practitioner contribute to the medical decision-making process. Health care providers explain treatments and alternatives to patients in order to provide the resources needed for patients to select the treatment option that best accords with their particular cultural and personal values.
Track 6: Drug research and development
A drug target is a naturally occurring cellular or molecular structure implicated in the pathophysiology of interest that the drug-in-development is intended to act on. It is the process of discovering a new drug and bringing a drug to market when a lead molecule has been found through the drug discovery process. Drug Discovery & advancement is now a cloud-based, intellectual solution that evaluates scientific information and data to uncover known, unknown, and hidden connectivity that can assist improve the huge possibilities of scientific advancement. It is utilised by pharmaceutical businesses, medical device companies, and academic institutions to help with new drug target identification and medication repurposing. Microbes battle for nutrition and dwelling space. Many microbes have acquired abilities to inhibit rival species from reproducing in order to live under these environments. Antimicrobial medications are mostly derived from microbes. The chemical structure must be determined in order to avoid the rediscovery of a chemical agent that is already recognised for its structure and chemical action.
Track 7: Patient-Centric clinical trials
Patient centricity will also hasten the innovation and enhancement of products, technology, and services used by patients in clinical trials. This is owing to the increased emphasis on real-time input. This, in turn, puts greater pressure on the industry to improve, move, and respond with greater agility than ever before. Not only has patient centricity changed the way clinical trials are designed, but it will also influence commercial and outsourcing decisions. Patient-centric medication development also provides a significant opportunity to define meaningful outcomes from the patient's point of view, ensuring that the needs and objectives of patient groups are reflected in research.
Track 8: Pharmacoepidemiology
Pharmacoepidemiology is the study of drug implementation and effects in large groups of people, and it produces an estimate of the probability of beneficial effects and the probability of bad effects in a population. It is a science that spans both clinical pharmacology and epidemiology. Descriptive epidemiology discusses disease and may include rate calculations. Drug usage studies are typically classified as descriptive studies. Analytic epidemiology researches are classified into two types: observational studies and experimental investigations. Clinical pharmacology is the study of the effects of medications on patients to determine the likelihood of undesirable effects on populations.
Track 9: Pharmacovigilance & drug safety
Pharmacovigilance is the science and detection of drug-related activities, as well as the assessment, comprehension, and prevention of adverse effects or any other drug-related problem. One of the main elements of adverse event reporting is the Individual Case Safety Report. The triage step of a possible adverse event report is critical in determining whether the "four elements" of a valid ICSR are present, which are an identifiable patient, an identifiable reporter, a suspicious drug, and an adverse event. A variety of strategies are used for signal detection. SD is a crucial component of medication use and safety monitoring. A safety signal is defined by the WHO as "reported information on a causal relationship between an adverse event and a drug, the relationship previously unknown or incompletely documented." A risk management strategy is a recorded plan that discusses the hazards associated with the use of a drug and how they are managed. The risks reported in an RMP are classified into three types: identifiable risks, possible risks, and unknown risks. Clinical trial reporting, also known as SAE (serious adverse event) reporting from clinical trials, safety information from clinical studies, is used to establish a drug's safety profile in humans and is a key component that drug regulatory authorities consider when deciding whether to grant or deny market authorization for a drug.
Track 10: Pharmacogenomics
Pharmacogenomics is the study of the genome in drug response. It investigates the impact of acquired and inherited genetic variation on drug response in patients by connecting gene expression or single-nucleotide polymorphisms with pharmacokinetics and pharmacodynamics. There are several identified genes that are significantly responsible for differences in medication metabolism and response. For the sake of brevity, the focus of this article will concentrate on the genes that are more frequently recognised and used clinically. They are Cytochrome P450s, VKORC1, and TPMT. Patient genotypes are typically classified as Ultra-rapid metabolizer, Extensive metabolizer, Intermediate metabolizer, or Poor metabolizer. Pharmacogenomics could help to limit the occurrence of polypharmacy. It is hoped that with personalised medicinal therapies, patients will not need to take multiple medications to treat the same ailment. Toxicogenomics is a branch of pharmacology that deals with the collecting, interpretation, and preservation of information about gene and protein activity inside a specific cell or tissue of an organism in response to toxic chemical exposure.
Track 11: Clinical research in oncology
Oncology is the study of tumours. Integrative oncology addresses the brain, body, and soul. That is why countless professionals, including medical specialists, have embraced the rapidly expanding field of integrative oncology, which combines the best of conventional and alternative therapies. Clinical trials in oncology have evolved to include phase I dosefinding trials, phase II studies to establish efficacy in a single tumour type, phase III trials comparing standards of care with potential advances in care, and phase IV studies to extend safety and activity data in a postmarketing scenario. Immuno-oncology treatments strengthen our immune system, allowing it to detect and eliminate cancer cells. Because cancer cells are not like other cells in the body, the immune system fights them when it recognises them. Neuro-oncology evaluates and treats patients with essential and optional cancers of the brain, spinal cord, and the layers surrounding the brain and spinal cord (meninges).
Track 12: Clinical research on stem cells and genetics
Stem cells are biological cells that can differentiate into other types of cells and divide to produce more of the same type of stem cell. They are found in multicellular organisms. Adult stem cells and progenitor cells serve as the body's repair system, renewing adult tissues. In mammals, there are two types of stem cells: embryonic stem cells, which are separated from the inner cell mass of blastocysts, and adult stem cells, which are found in diverse tissues. Because genetics is the essential base of any creature, studying genetics will provide a powerful technique of discovering hereditary factors in illness aetiology. In recent years, genetic research has shifted from disorders caused by a single gene (for example, Huntington's disease) to common multi-factorial disorders (for example, hypertension) caused by interactions between inherited gene variants and environmental factors such as chemical, physical, biological, social, infectious, behavioural, or nutritional factors. Molecular genes were known to exist on chromosomes; however, chromosomes are made up of both protein and DNA, and scientists were unsure which of the two is in charge of heredity.
Track 13: Medical and clinical case reports
Medical Case Reports provides a concentrated valuable collection of cases in various disciplines, making it easy for healthcare practitioners, academics, and others to access clinically essential information about common and rare disorders. The journal primarily focuses on patient disease symptoms, signs, diagnosis, therapy, and follow-up in many domains. Diabetes case study- Diabetes is a chronic metabolic condition that arises when the human body is unable to create enough insulin or when cells do not respond to insulin produced. High blood sugar causes symptoms such as frequent urination, thirst, and hunger. Cancer case reports- An estimated 14.1 million new cancer cases occurred worldwide in 2012. More than four out of every 10 cancers occur in nations with low or medium Human Development Indexes.
Track 14: Clinical study design advancements
The formulation of trials and experiments, as well as observational studies, in medical, clinical, and other sorts of research involving humans is known as clinical study design. A clinical study's purpose is to evaluate the safety, efficacy, and mechanism of action of an investigational medical product or procedure, or novel medicine or technology that is in development but has not yet been approved by a health authority. It can also be used to explore a medicine, equipment, or technique that has already been approved but requires additional research, generally in terms of long-term effects or cost-effectiveness. A randomised controlled trial is a form of scientific experiment that seeks to eliminate bias when assessing a novel treatment. An adaptive clinical trial is a clinical study that examines a medical device or treatment by observing participant results on a predetermined schedule and altering trial protocol parameters in accordance with those observations.
Track 15: Globalization of clinical trials
The globalisation of clinical research is a relatively new phenomenon in which many of these studies are being conducted on a global basis, with a major increase in clinical trials being conducted in developing nations. From 2005 to 2012, the Asian (30%) and Latin American/Caribbean (12%) regions had the highest average yearly growth rates in clinical trials; other geographic regions had growth rates lower than the global average (8%). Lower-middle income (33%) and low-income (21%) regions saw the highest average annual growth. Emerging economies from low-middle income countries experienced the most country-specific growth, followed by South Korea, Japan, India, Brazil, and Turkey. With the globalisation of clinical trials, it is vital to strengthen legal and ethical criteria to ensure the integrity of study participants. Observers remarked more than a decade ago that research was being conducted in impoverished countries with no respect for international ethical guidelines. The process of globalisation of clinical trials can thus be advantageous because, for example, it provides participants with access to new treatments; however, it requires discussion and monitoring of ethical questions related primarily to ensuring the integrity, welfare, and safety of the research participant; to bioethical frames of reference, such as autonomy, beneficence, justice, and fairness.
Track 16: Future & innovations of clinical trials
Clinical studies for the advancement of novel drugs are typically initiated and funded by industry. There are also numerous clinical studies that have been initiated by educated clinical scientists. Clinical research, whether initiated by industry or by academic clinical examiners, is frequently carried out in national, European, and global consortia, which can occasionally be large. Clinical research raises serious moral and security concerns. The safety of participants in a clinical trial is critical. As a result, clinical research is extremely supervised. Many components of this control are combined at the European level as well as around the world to encourage cooperative actions across borders.
Track 17: Bioinformatics-Based Clinical Research
Bioinformatics, a blend of biotechnology, genetics, and data innovation, investigates and even quantifies information. It even plays a significant role in understanding the subatomic processes that underpin life. Clinical bioinformatics is the name given to the branch of bioinformatics that deals with clinical preliminary data. Understanding the link between clinical informatics and bioinformatics aids in the discovery and development of new diagnosis and treatments for infections. The therapeutic use of bioinformatics is linked to research and innovation in order to comprehend subatomic instruments and potential treatments for human illnesses.
Track 18: Post Marketing Surveillance
Post marketing surveillance is an important component of the science of pharmacovigilance, which entails monitoring the safety of pharmaceutical goods and medical devices after they are introduced to the market. Because clinical trials—involving relatively few individuals who typically do not have additional medical conditions that exist in the general population—are the foundation for the acceptance and approval of drugs and medical devices, post-marketing surveillance can further hone, or confirm, the safety of a drug or device after it has been used in the general population by numerous individuals with a variety of medical conditions.
Market Analysis
CLINICAL TRIALS MARKET OVERVIEW
The global clinical trials market was worth USD 56,640.7 million in 2022 and is expected to rise to USD 99,212.0 million by 2030. The clinical trials market is estimated to grow at a 7.4% CAGR from 2023 to 2030. The clinical trials market is an important component of the healthcare business since it entails conducting clinical studies to assess the safety and efficacy of novel medications, medical equipment, and therapies.
The market covers several stages, such as clinical trial design, conduct, and analysis.
In clinical trials, there is a rising emphasis on a patient-centric approach, which includes incorporating patients in trial design and providing them more influence over their involvement. This method can help to improve patient happiness, increase patient retention, and create more accurate data.
Decentralised clinical trials entail performing clinical trials in a patient's home or community environment rather than at a clinical trial centre. This method can improve patient recruitment, lower costs, and promote patient engagement.
MARKET DYNAMICS AND TRENDS IN CLINICAL TRIALS
The growth of the global clinical trial market is being driven by an increase in R&D spending.
The rising R&D spending of major biopharmaceutical companies is propelling the global clinical trials market forward. Furthermore, major biopharma businesses intend to make significant cumulative investments in 2023 to support launch outcomes and R&D projects that are expected to conduct long-term growth endeavours. For example, Pfizer's adjusted spending projection for R&D in 2023 is USD 12.4 to USD 13.4 billion, up from USD 3,615 million in 2022, implying a 4% increase.
The high cost and low success rate of clinical trials are impeding the global clinical trial market's growth.
According to a research submitted to the US Department of Health and Human Services, the moderate cost of phase 1, 2, and 3 clinical trials across therapeutic domains is around USD 4, 13, and 20 million, respectively. Furthermore, according to the American Council on Science and Health, the success rate varies greatly depending on the therapeutic area.
MARKET SEGMENT ANALYSIS FOR CLINICAL TRIALS
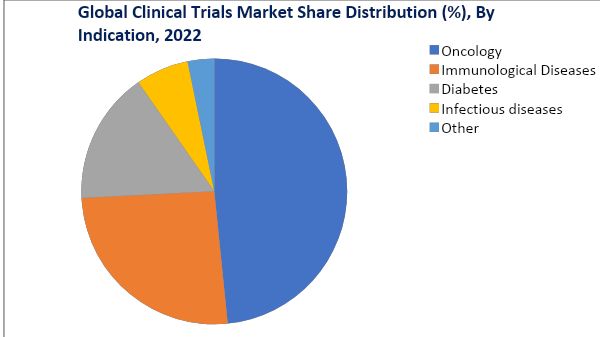
The Oncology Segment is Expected to Hold a Dominant Position in The Market Over The Forecast Period.
The growing number of clinical trials for cancer treatment indicates that the oncology segment dominates the global clinical trials market by withholding around 28.8%.
For instance, according to clinicaltrials.gov, for cancer treatment, there were around 106 early phase-I interventional trials active, 2478 phase-I interventional trials were active, 4219 phase-II interventional trials were active, 2031 phase-III interventional trials were active, and 585 phase-IV interventional trials were active in 2022.
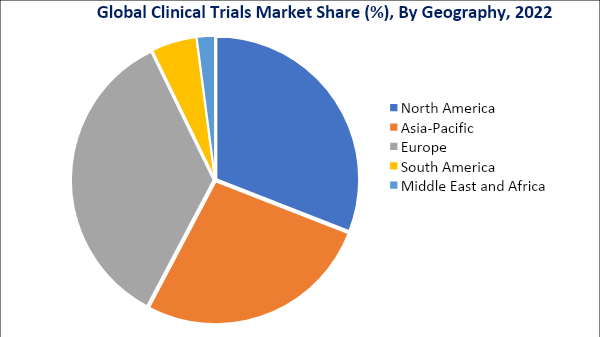
CLINICAL TRIALS MARKET GEOGRAPHICAL PENETRATION
North America Holds the Largest Share of the Global Clinical Trials Market.
The increasing number of clinical trials registered in North America occupied the highest clinical trials market share of about 40.3% in 2022 which is expected to increase to 40.7% by 2030.