Theme: “Innovative advancements in global clinical research and clinical trialsâ€
Euro clinical trials 2019
Clinical research & clinical trials: academic perspective
Clinical research is branch of medical science that discover the efficacy and accuracy of medicine, medical devices and treatment. This includes systemic, observational and experimental biomedical studies. Clinical research plays a major role in the prevention of disease state, treatment, diagnosis and cure of various health disorders. This involves a period of events from pre-clinical animal testing to several stages of drug development. Pre-clinical reviews and non-clinical studies is a phase of pre-clinical research that starts before clinical trials. Treatment research includes an intervention they are medication, psychotherapy, new approaches to surgery. Prevention research looks for more effective ways to prevent diseases and disorders. Diagnostic research is the practice of expecting for more effective ways to identify disorder condition. Screening research is to find the better ways to detect several diseases and health disorders. Quality of life trials ways to enhance comfort and quality of life for individuals with a chronic illness. Genetic studies target to upgrade the forecast of disorders by establishing and comprehension how genes and illness may be relate.
Clinical trials conduct
Clinical trials are observations on clinical research. Prospective behavioural research studies on human beings to answer particular queries on biomedical or behavioural interventions. This creates data on safety and efficacy. In master protocol, multiple experimental treatments are tested in only one trail. Clinical trial protocol is used to describe and supervise the trail. This protocol contains a definite study plan to assure safety and health trails and to give a correct template for trail conduct to inspector. A clinical trial involves new drugs are classified into five phases. Each phase of the drug compliance is treated as a separate clinical trial. The drug-development process will move forward through all four phases. Phase 0 – pharmacodynamics and pharmacokinetics in humans (testing within 10 to 15 people). Phase 1 – screening for safety (testing within a small group of people 20 to 80 people). Phase 2 – establishing the efficacy of the drug (testing with a larger group of people 100 to 300 people). Phase 3 – final confirmation of safety and efficacy (testing with a large group of people 1000 to 3000 people). Phase 4 –safety studies during sales (post marketing studies describes additional information including the treatments risk, benefits, and optimal use).
Clinical data management
Clinical data management assures collection, integration and availability of data at acceptable quality and cost. It leads to the making of high-quality, reliable, and statistically sound data from clinical trials. The data management plan explains the activities to be conducted in the processing data and used for description of data sources, data handling process and data control procedures. Clinical trials using an electronic CRF database design and also using paper CRF. Validation rules are electronic analysis explains in advance which make sure the integrity and uniformity of the clinical trial data. An electronic CRF is used data entry is drifting out towards the inspector site where the clinical trials are conducted. Samples which are collected during clinical trial they are sent to be a single central laboratory analysis. The CRF collects adverse events disclosed during the conduct of the clinical trial, there is a separate process which make sure that serious adverse events are disclosed quickly. The clinical data manager must make sure that data is reconciled between these processes.
Clinical research on different diseases
Clinical Trials for various substances and disorders are directed for evaluating at least one medication for treating an infection, disorder and moreover discovering appealing to keep the improvement or repeat of an illness or condition. This includes solutions, antibodies, or way of life changes, among different methodologies. Clinical trials on behaviours, mental, eating and sleeping disorders. Clinical trials for diabetes and cardiovascular diseases, Clinical trials for Alzheimer’s, Trials on pulmonary/respiratory diseases, Research and studies on wounds and injuries, Acquired immune deficiency syndrome, Gynaecological Infections, Infectious Colitis and HIV Infections.
Ethics in clinical research
Research ethics includes the fundamental ethical principles of various topics involving research and scientific research. Medical ethics is a system of moral principles that apply values to the practice of clinical medicine and in scientific research. Medical ethics is based on a set of values that professionals can refer to in the case of any confusion or conflict. Privacy for research participants is a concept in research ethics which states that a person in human subject research has a right to privacy when participating in research. Shared decision-making in medicine (SDM) is a process in which both the patient and physician contribute to the medical decision-making process. Health care providers explain treatments and alternatives to patients to provide the necessary resources for patients to choose the treatment option that best aligns with their unique cultural and personal beliefs.
Drug discovery & development
Drug target is the naturally existing cellular or molecular structure involved in the pathology of interest that the drug-in-development is meant to act on. It is the process to discover a new drug and bringing a drug to the market once a lead compound has been identified through the process of drug discovery. Drug Discovery & Development is these days a cloud-based, intellectual solution which analyses scientific knowledge and data to unveil known, unknown & hidden connectivity that can help increase the vast possibilities of scientific development. It is used by pharmaceutical companies, medical device companies and academic institutions to assist with new drug target identification and drug re-purposing. Microbes compete for living space and nutrients. To survive in these conditions, many microbes have developed abilities to prevent competing species from proliferating. Microbes are the main source of antimicrobial drugs. The elucidation of the chemical structure is critical to avoid the re-discovery of a chemical agent that is already known for its structure and chemical activity.
Patient-Centric clinical trials
Patient centricity will also bring about faster evolution and improvement of the products, technologies and services being utilized by patients in clinical trials. This is due to the more real-time feedback culture. This will in turn create industry pressure for improvement, movement and more agility in responsiveness than ever before. Not only has patient centricity disrupted the way we design clinical trials but it will also disrupt its business and outsourcing decisions. Patient-centric drug development also offers a huge opportunity to define meaningful outcomes from the patient perspective, as a way to ensure the needs and priorities of patient populations are reflected in research.
Pharmacoepidemiology
Pharmacoepidemiology is the study of the implementation and effects of drugs in many numbers of people and it produces an estimate of the probability of beneficial effects of a drug in a population and the probability of adverse effects. It can be called a bridge science spanning both clinical pharmacology and epidemiology. Descriptive epidemiology explains disease and may consist of calculating rates. Studies of drug use would generally fall under descriptive studies. Analytic epidemiology contains two types of studies they are observational studies and experimental studies. Clinical pharmacology is to produce a risk benefit assessment by effects of drugs in patients that assessing the probability of adverse effects on populations.
Pharmacovigilance & drug safety
Pharmacovigilance is the science and detection of relating activities, assessment, understanding and prevention of adverse effects or any other drug-related problem. Individual Case Safety Report is one of the fundamental principles of adverse event reporting. The triage phase of a potential adverse event report, it has the great significance to determine the "four elements" of a valid ICSR are present they are -an identifiable patient, an identifiable reporter, a suspect drug, and an adverse event. Signal detection includes a range of techniques. SD is an essential part of drug use and safety supervision. The WHO defines a safety signal as: "Reported information on a causal relationship between an adverse event and a drug, the relationship being unknown or incompletely documented previously". A risk management plan is a documented plan that explains the risks occurred with the use of a drug and how they are being controlled. The risks described in an RMP fall into one of three categories: identified risks, potential risks, and unknown risks. Clinical trial reporting also known as SAE (serious adverse event) reporting from clinical trials, safety information from clinical studies is used to establish a drug's safety profile in humans and is a key component that drug regulatory authorities consider in the decision-making as to whether to grant or deny market authorization for a drug.
Pharmacogenomics
Pharmacogenomics is the study of genome in drug response. It deals with the influence of acquired and inherited genetic variation on drug response in patients by correlating gene expression or single-nucleotide polymorphisms with pharmacokinetics and pharmacodynamics. There are several known genes which are largely responsible for variances in drug metabolism and response. The focus of this article will remain on the genes that are more widely accepted and utilized clinically for brevity. They are Cytochrome P450s, VKORC1, and TPMT. Patient genotypes are usually categorized into Ultra-rapid metabolizer, Extensive metabolizer, Intermediate metabolizer, Poor metabolizer. A potential role pharmacogenomics may play would be to reduce the occurrence of polypharmacy. It is theorized that with tailored drug treatments, patients will not have the need to take several medications that are intended to treat the same condition. Toxicogenomics is a sub discipline of pharmacology that deals with the collection, interpretation, and storage of information about gene and protein activity within a particular cell or tissue of an organism in response to exposure to toxic substances.
Oncology clinical research
Oncology is a branch of arrangements with tumours. Integrative oncology deals with the brain, body, and soul. That is the reason numerous experts, including medicinal specialists, have grasped the quickly extending field of integrative oncology, which merges the best of customary and option medications. The science of clinical trials in oncology evolved to include phase I doseâ€finding trials, phase II studies to establish efficacy in a single tumour type, phase III trials comparing standards of care with potential advances in care, and phase IV studies to extend safety and activity data in a postâ€marketing scenario. Immuno-oncology treatments enact our invulnerable framework, making it ready to perceive growth cells and destroy them. Since tumour cells are altogether different from ordinary cells in the body, the resistant framework attacks them when it can remember them. Neuro-oncology assesses and treats individuals with essential and optional tumours of the cerebrum, spinal line, and the layers encompassing the mind and spinal rope (meninges).
Stem cell & Genetic clinical research
Stem cells are biological cells that can differentiate into other types of cells and can divide to produce more of the same type of stem cells. They are found in multicellular organisms. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing adult tissues. In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and adult stem cells, which are found in various tissues. Genetics is the fundamental basis of any organism so understanding of genetics will provide a powerful means to discover hereditary elements in disease etiology. In recent years, genetic studies have shifted from disorders caused by a single gene (e.g. Huntington’s disease) to common multi-factorial disorders (e.g. hypertension) that result from the interactions between inherited gene variants and environmental factors, including chemical, physical, biological, social, infectious, behavioural or nutritional factors. Molecular genes were known to exist on chromosomes, chromosomes are composed of both protein and DNA, and scientists did not know which of the two is responsible for inheritance.
Medical and clinical case reports
Medical Case Reports delivers a focused valuable collection of cases in all disciplines so that healthcare professionals, researchers and others can easily find clinically important information on common and rare conditions. The journal mainly focuses on symptoms, signs, diagnosis, treatment, and follow-up of patient disease in different areas. Diabetes case report- Diabetes is a chronic metabolic disease that occurs when the human body is not able to produce enough of the hormone insulin or because cells do not respond to the insulin that is produced. High blood sugar produces symptoms of frequent urination, increased thirst and hunger. Cancer case reports- In 2012, an estimated 14.1 million new cases of cancer occurred worldwide more than 4 in ten cancers occurring worldwide are in countries at a low or medium level of Human Development Index.
Innovations in clinical study designs
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. The goal of a clinical study is to assess the safety, efficacy, and the mechanism of action of an investigational medicinal product or procedure, or new drug or device that is in development, but potentially not yet approved by a health authority. It can also be to investigate a drug, device or procedure that has already been approved but is still in need of further investigation, typically with respect to long-term effects or cost-effectiveness. A randomized controlled trial is a type of scientific experiment which aims to reduce bias when testing a new treatment. An adaptive clinical trial is a clinical trial that evaluates a medical device or treatment by observing participant outcomes on a prescribed schedule, and modifying parameters of the trial protocol in accord with those observations.
Globalization of clinical trials
The globalization of clinical research is a relatively recent phenomenon, in which many of these studies are taking place on a global scale, with a significant increase of clinical trials in developing countries. The largest clinical trials average annual growth from 2005–2012 occurred in Asian (30%), and Latin American/Caribbean (12%) regions; other geographic regions had growth rates less than the world average (8%). The largest average annual growth occurred in lower-middle income (33%) and low-income (21%) regions. Emerging economies from low-middle income countries had the largest country-specific growth; other countries included South Korea, Japan, India, Brazil, and Turkey. With the globalization of clinical trials, it becomes necessary to strengthen legal and ethical guidelines for guaranteeing the research participants‟ integrity. Some observers noted, more than a decade ago, that studies were being run in developing countries without concerns regarding adherence to the international ethical principles. The process of globalization of clinical trials, therefore, can be advantageous because, for example, it gives to access to new treatments to participants; however, it requires discussion and the monitoring of ethical questions related mainly to ensuring the integrity, welfare and safety of the research participant; to the frames of reference of bioethics, such as autonomy, beneficence, justice and fairness.
Future & innovations of clinical trials
Clinical trials for the advancement of new medications are and their most part started and financed by industry. There are additionally numerous clinical trials started by scholarly clinical scientists. Whether started by industry or by scholastic clinical examiners clinical research is frequently performed in national, European and overall consortia, which can now and then, be expansive ones. Clinical research brings up significant moral and security issues. The security of members in a clinical trial is of vital significance. As an outcome, clinical research is exceedingly controlled. To encourage coordinated efforts crosswise over fringes, numerous parts of this control are blended at the European level additionally around the world.
Clinical pharmacy and pharmacy community
Clinical pharmacy is the branch of pharmacy in which doctor of pharmacy provide patient care that optimizes the use of medication and promotes health, wellness, and disease prevention. Clinical pharmacists care for patients in all health care settings but the clinical pharmacy movement initially began inside hospitals and clinics. Clinical pharmacists often work in collaboration with physicians, nurse practitioners, and other healthcare professionals. Clinical pharmacists are a primary source for valid information and advice regarding the safe, appropriate, and cost-effective use of medications. A pharmacy is the place where most pharmacists practice the profession of pharmacy. Community pharmacies usually consist of a retail store front with a dispensary where medications are stored and dispensed. Pharmacies are typically required to have a pharmacist on-duty at all times when open. It is also often a requirement that the owner of a pharmacy must be a registered pharmacist.
Chronic disease state management
Chronic diseases are long-term conditions that usually progress slowly over time. This includes conditions such as heart disease, diabetes, chronic kidney disease, asthma and chronic obstructive pulmonary disease (COPD), depression, and arthritis, among many others. Chronic Disease Management (CDM) is on-going care and support to assist individuals impacted by a chronic health condition with the medical care, knowledge, skills and resources they need to better manage on a day to day basis. This may include regular visits and support from your family physician, other primary care provider, community-based programs or referrals to specialist programs and services.
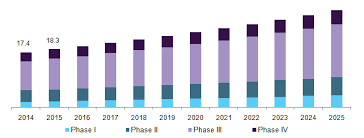
The global clinical trials market is expected to reach USD 65.2 billion by 2025, according to a new report by Grand View Research, Inc. Key drivers impacting the market growth are globalization of clinical trials, development of new treatments such as personalized medicine, augmenting evolution in technology, and boosting demand for CROs to conduct clinical trials. Globalization of clinical trial has led to increase in investment in new product development in emerging countries thereby, having a positive impact on overall market. The availability of the vast array of services from drug discovery to post-marketing surveillance has further simplified the life for mid-size and small-scale pharmaceutical and biotechnological organizations by providing them the option to outsource what they think is beyond their core expertise. For instance, Pfizer currently has three CROs working with it to enhance its product portfolio and drive innovation. According to the partnership agreement with ICON in 2011, Pfizer would only preserve the scientific ownership for the trials and studies conducted by ICON, hence allowing the company to focus and further develop its capabilities in clinical trial designing.
Growing prevalence of disease and incidence of new disease is expected to give further boost to the clinical trial market. Worldwide population has varied disease profile with emerging countries having the most diverse disease profile. This is expected to boost the clinical trial of new or rare disease which otherwise would not have found any sponsors. More number of patients having a specific disease would act as a stimulus for biopharmaceutical companies to invest more in clinical trials for a disease segment.
U.S. clinical trials market, by phase, 2014 - 2025 (USD Billion)
For More details visit: https://clinicaltrials.pharmaceuticalconferences.com/
Conference Highlights
- Clinical Research & Clinical Trials: Academic Perspective
- Clinical Trials Conducts
- Clinical Data Management
- Clinical Research on different diseases
- Ethics in Clinical Research
- Drug discovery & development
- Patient-Centric Clinical Trials
- Pharmacoepidemiology
- Pharmacovigilance & Drug safety
- Pharmacogenomics
- Oncology Clinical Research
- Stem cell & Genetic clinical research
- Medical and Clinical case reports
- Innovations in Clinical study designs
- Globalization of Clinical Trials
- Future & innovations of Clinical Trials
- Clinical Pharmacy and Pharmacy Community
- Chronic disease state management
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | March 18-19, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Trials
- Journal of Clinical Research & Bioethics
- Journal of Clinical Case Reports
Abstracts will be provided with Digital Object Identifier by




















